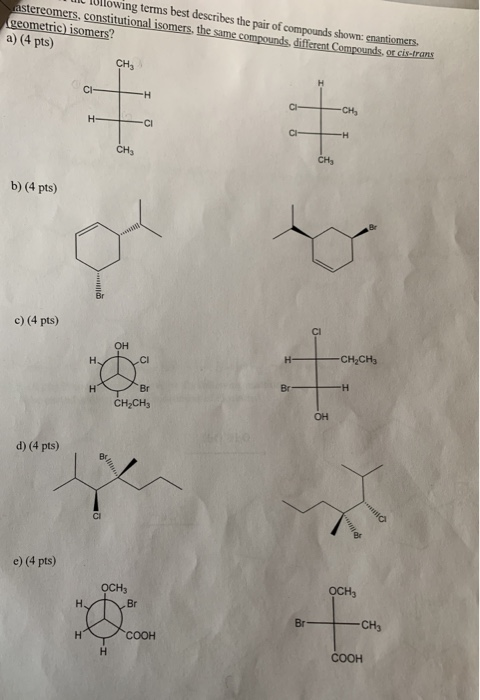
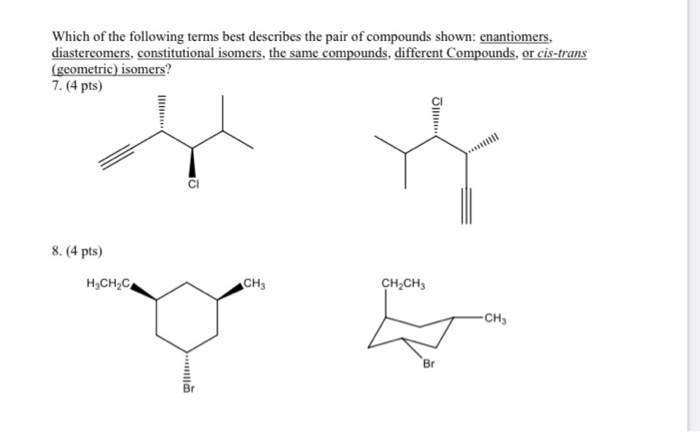
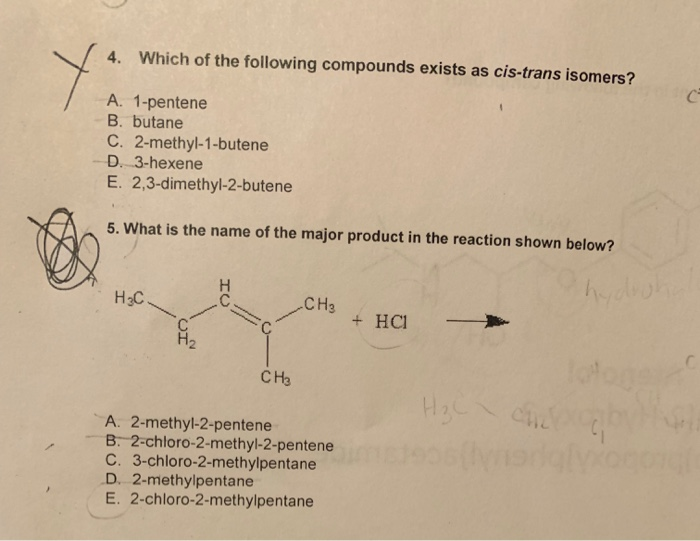
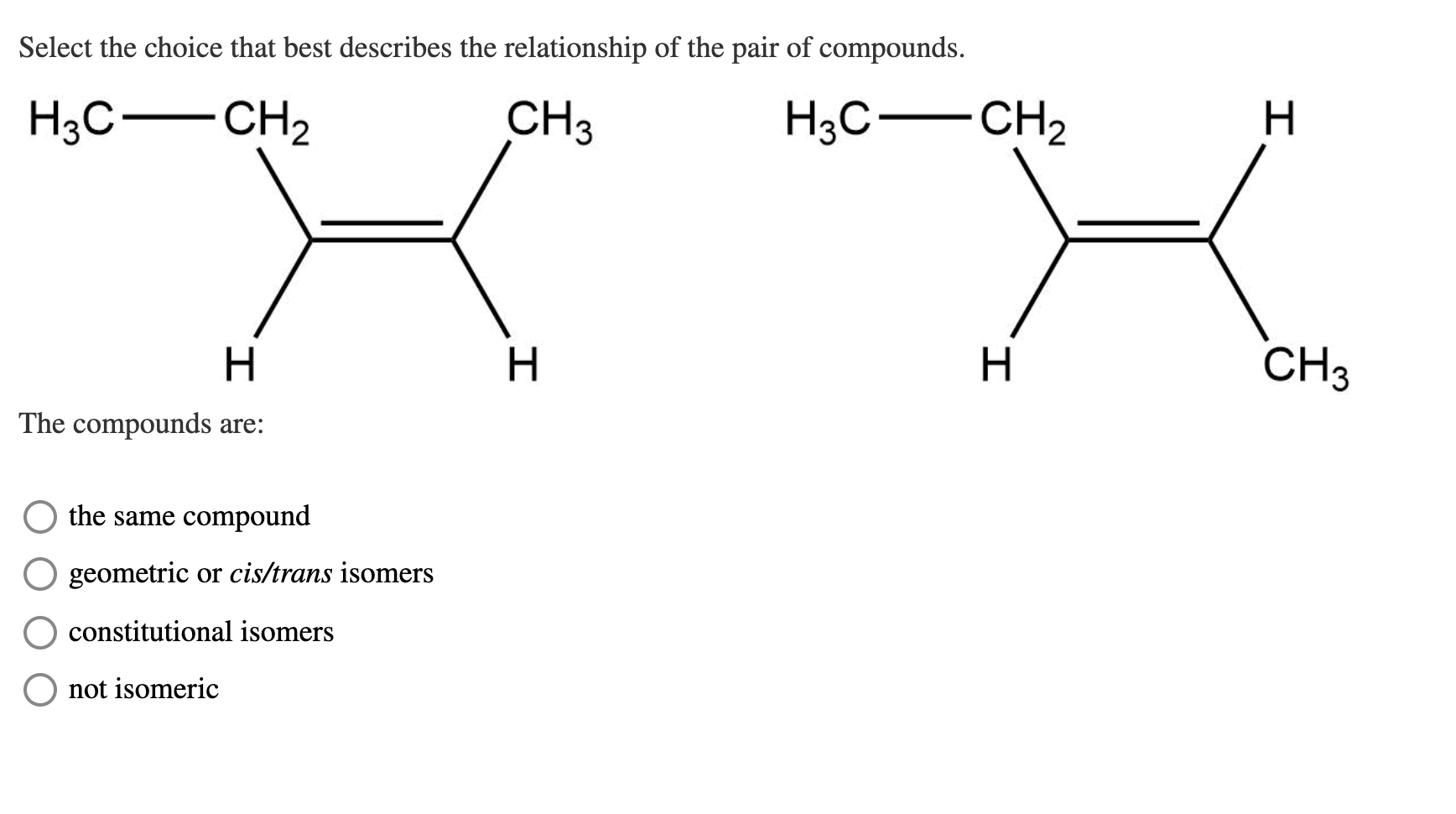
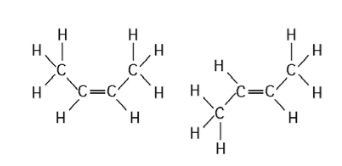
Which Of The Following Best Describes Cis Trans Isomers
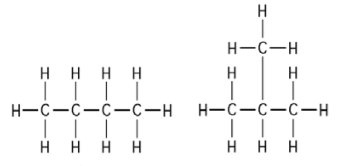
Which of the following best describes cis trans isomers. BIOL 1408 SAPLING CH 2 HW. A They have variations in arrangement around a double bond. They are long chains of hydrogen and carbon atoms.
Trans has no or very less dipole moment than cis isomer. Maleic acid is the cis isomer and fumaric acid is the trans isomer. D They have different molecular formulas.
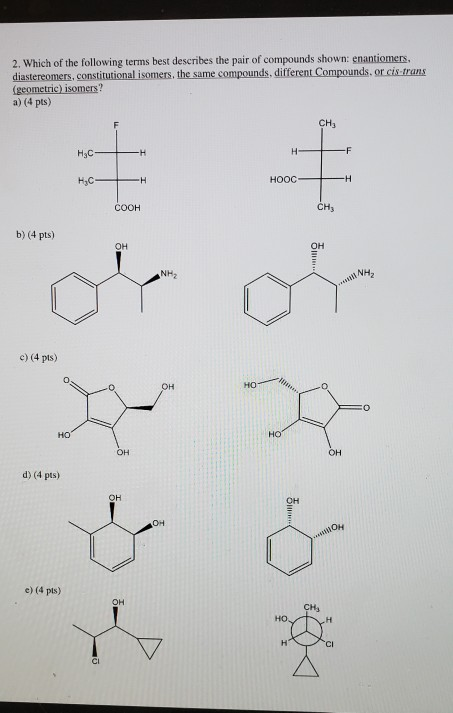
E They have variations in arrangement around a double bond. B They have an asymmetric carbon that makes them mirror images. They differ in the arrangement of covalent bonds and in covalent partners.
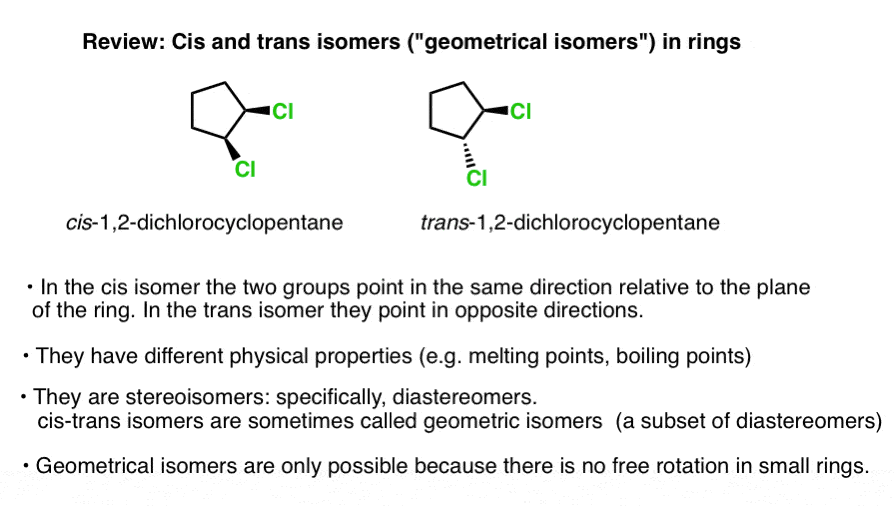
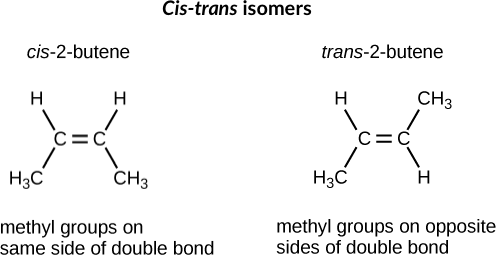
Which of the following best describes cis-trans isomers. The isomer with the two Cl atoms on opposite sides of the molecule is the trans isomer Latin trans meaning across and is named trans -12-dichloroethene. The isomer in which the two chlorine Cl atoms lie on the same side of the molecule is called the cis isomer Latin cis meaning on this side and is named cis-12-dichloroethene.
Which of the following statements correctly describes cis-trans isomers. The former is solid at room temperature melting point 43 o C and the latter is found to be liquid with a melting point of 134 o. Elaidic acid and oleic acid are cis-trans isomers.
Cis-trans isomerism can be found when the position of a side group is changed while the rest of the molecules are identical to each other. This organic chemistry video tutorial provides a basic introduction into cis and trans isomers using alkenes and cycloalkanesSubscribehttpswwwyoutubec. B Their atoms and bonds are arranged in different sequences.
Cis isomer has more dipole moment than trans isomer because it has two similar groups on same side of double bond. C They have the same chemical properties.
32 Which of the following statements correctly describes cis-trans isomers.
They have the same number of atoms of the same elements but different structures. They are long chains of hydrogen and carbon atoms. B They have an asymmetric carbon that makes them mirror images. Which of the following best describes cis-trans isomers. E Their atoms and bonds are arranged in different sequences. E They have variations in arrangement around a double bond. Cis-trans isomerism can be found when the position of a side group is changed while the rest of the molecules are identical to each other. This organic chemistry video tutorial provides a basic introduction into cis and trans isomers using alkenes and cycloalkanesSubscribehttpswwwyoutubec. Which of the following statements correctly describes cis-trans isomers.
A They have variations in arrangement around a double bond. They differ in their spatial arrangement around inflexible double bonds. They differ in their constituent atoms. They differ in their connectivity among their component atoms. B Their atoms and bonds are arranged in different sequences. They are long chains of hydrogen and carbon atoms. They are mirror images of each other.

















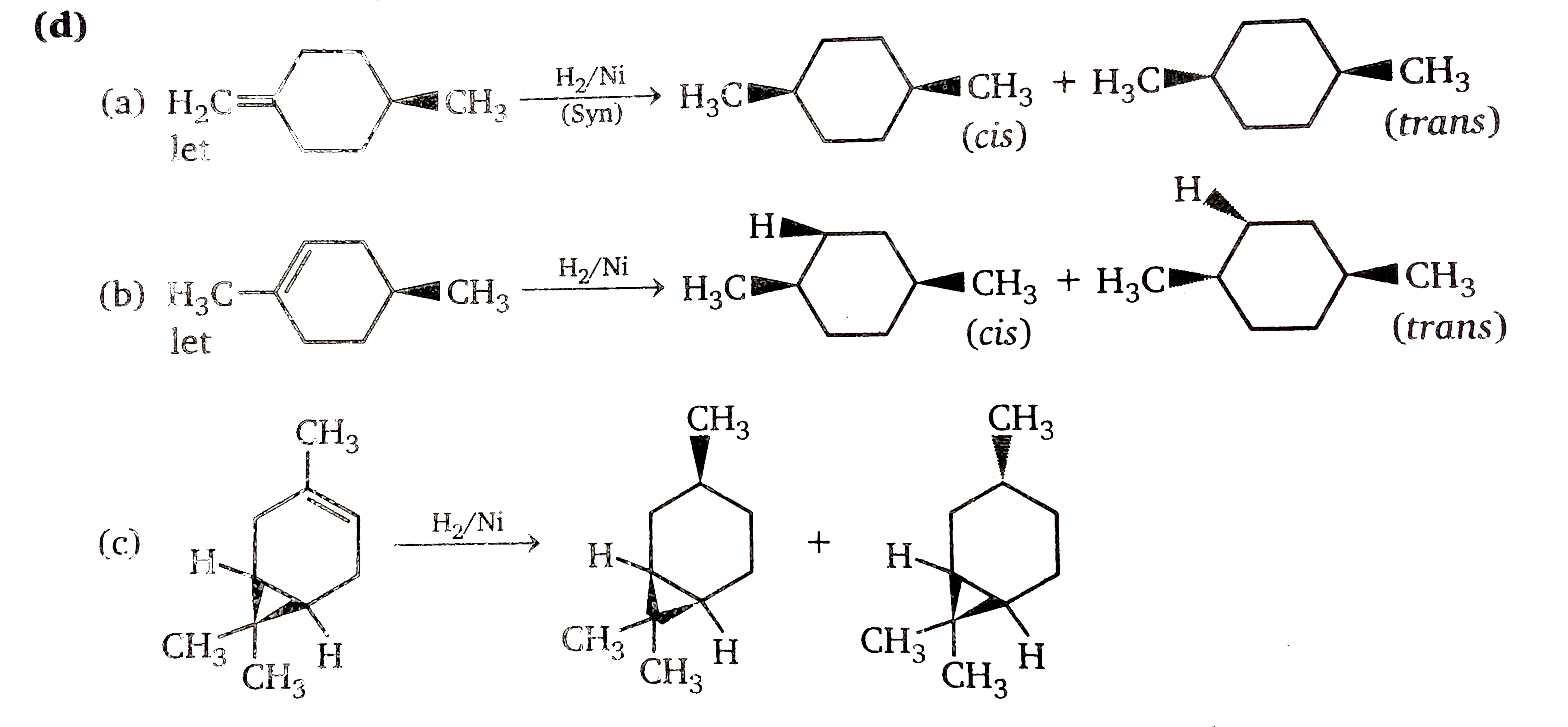







Post a Comment for "Which Of The Following Best Describes Cis Trans Isomers"